What is Market access in Pharmaceutical industry?
Market access in the pharmaceutical sector encompasses a multifaceted approach that involves addressing regulatory requirements, negotiating pricing and reimbursement agreements, and tailoring strategies to meet the diverse needs of patients and healthcare systems.
How can companies benefit from Market access in pharma?
Effective market access strategies enable pharmaceutical companies to penetrate new markets and expand their reach. By securing regulatory approvals, negotiating pricing and reimbursement agreements, and addressing payer requirements, companies can gain access to larger patient populations and drive growth.
One innovative approach involves leveraging real-world data and evidence to demonstrate the value of pharmaceutical products. By conducting robust real-world studies and health economic analyses, companies can provide compelling evidence of a product’s efficacy, safety, and cost-effectiveness. This data-driven approach not only strengthens the case for reimbursement but also enhances confidence among healthcare providers and payers. In a highly competitive industry, strong market access capabilities can provide a significant competitive advantage. Companies with well-executed strategies are better positioned to differentiate themselves from competitors and capture market share.

Market access to Central Eatern Europe and Adriatic region with LENIS
LENIS’s expansion into the Central and Eastern European (CEE) region, along with its venture into the Adriatic region, with its market access solutions in the pharmaceutical industry marks a strategic move that underscores the company’s commitment to enhancing patient access to innovative therapies across diverse markets.
LENIS’s presence in the CEE and Adriatic regions signifies its recognition of the unique challenges and opportunities presented by these markets. By offering strategies that align with local regulations, payer dynamics, and patient needs, LENIS aims to facilitate the availability and affordability of its pharmaceutical products in these regions.
One of the key strengths of LENIS lies in its expertise in navigating regulatory complexities, which is particularly crucial in markets like those in the CEE and Adriatic regions where regulatory frameworks may vary significantly from those in Western Europe or North America. LENIS’s regulatory affairs team is well-equipped to ensure compliance with local regulations and secure timely approvals for its products, enabling swift market entry.
By conducting thorough market analyses and leveraging health economics and outcomes research (HEOR), LENIS negotiates favorable reimbursement agreements with payers in both the CEE and Adriatic regions, optimizing market access and uptake of its portfolio of products. This comprehensive approach underscores LENIS’s commitment to improving patient care standards and access to innovative therapies across the CEE and Adriatic regions.
Market access coverage in EU and non-EU countries
With a direct presence and established field force in Slovenia, Croatia, Bosnia and Herzegovina, Serbia, North Macedonia, Kosovo, Montenegro, and Albania, LENIS successfully manages this complex region (2 EU & 6 non-EU countries) for our partners.
In addition to the Adriatic region, LENIS has demonstrated its capacity and established network to encompass other smaller EU countries within a single distribution agreement. Under the coordination of LENIS managers, projects are seamlessly executed through collaboration with distribution partners across various countries.
LENIS has successfully established such arrangements for a diverse array of regions, including the Central and Eastern European (CEE) countries such as Hungary, Czech Republic, Slovakia, Romania, Bulgaria, and Poland. Furthermore, LENIS extends its reach to encompass the Baltic and Nordic states, ensuring comprehensive coverage across these territories.
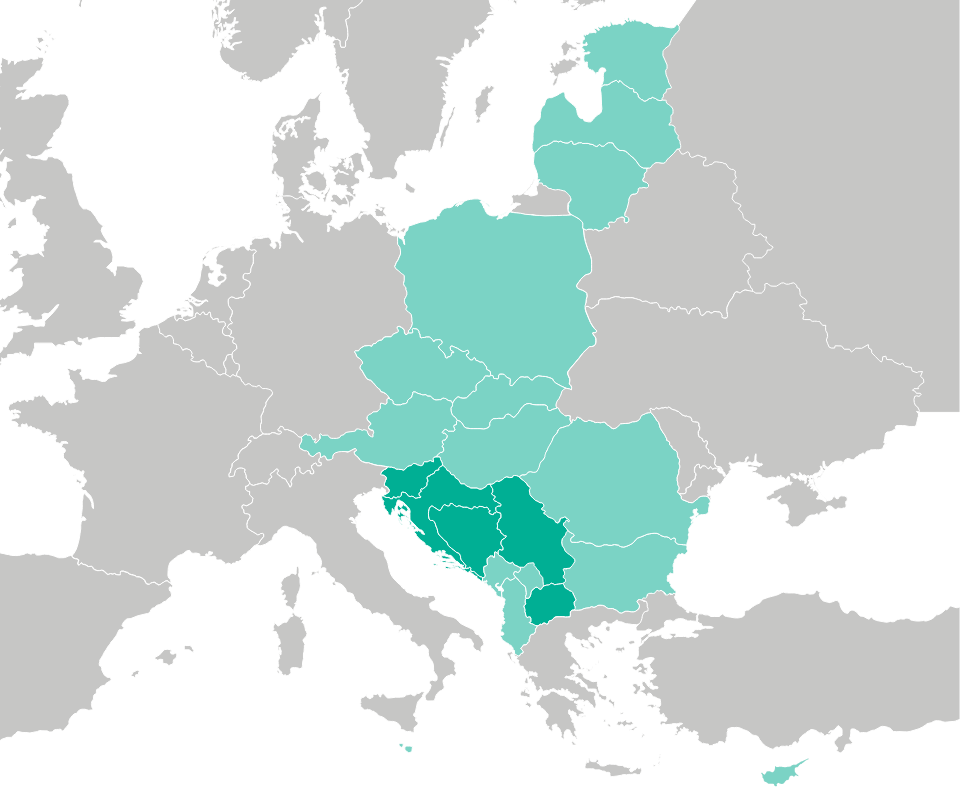
(Dark green color) Own affiliates, (Light green color) Stable partnerships
How does Market access propel success in the Pharmaceutical Industry?
Market access in pharma offers numerous benefits to companies, serving as a gateway to success and sustainability. It enables companies to penetrate diverse markets, reaching a wider patient base and increasing product demand. By securing reimbursement agreements with payers, companies can ensure that their products are accessible and affordable to patients, thus driving sales and revenue growth. Effective strategies enhance a company’s competitive advantage by differentiating its products in the market. By demonstrating the value of their therapies through health economics and outcomes research (HEOR), companies can justify their pricing and position themselves as leaders in their respective therapeutic areas.
Forging strategic partnerships and collaborations with healthcare providers, payers, and other stakeholders is essential for companies in the pharmaceutical industry. By fostering strong relationships with key players in the healthcare ecosystem, companies can gain valuable insights, access new opportunities, and drive innovation. Initiatives in pharma contribute to improving patient outcomes and quality of care. By ensuring that patients have access to the medications they need, companies can make a positive impact on public health and enhance their reputation as socially responsible entities.
How significant is Market access in pharma for the Central Eastern Europe and Adriatic Regions?
The market access landscape in the Central Eastern Europe (CEE) and Adriatic (Adria) regions is significant and continually evolving. These regions represent dynamic markets with diverse healthcare systems, regulatory frameworks, and patient populations.
In recent years, the pharmaceutical industry has increasingly focused on expanding its presence in CEE and Adriatic countries due to factors such as rising healthcare expenditures, increasing demand for innovative treatments, and evolving regulatory environments. Additionally, these regions offer opportunities for market growth and expansion, with growing populations and increasing awareness of healthcare needs.
In CEE and Adriatic countries it’s crucial for pharmaceutical companies to introduce their products to these markets successfully. Companies must navigate complex regulatory processes, negotiate pricing and reimbursement agreements with payers, and tailor their strategies to meet the specific needs of each country within these regions.
